 As the opioid addiction crisis grew to epidemic proportions, Georgia Tech alumnus and entrepreneur Ashley Hancock saw an opportunity to save lives while working with a physician and an addiction specialist. They have developed a medical device that can help patients adhere to prescribed dosages by controlling access to their medication and dispensing it properly.
As the opioid addiction crisis grew to epidemic proportions, Georgia Tech alumnus and entrepreneur Ashley Hancock saw an opportunity to save lives while working with a physician and an addiction specialist. They have developed a medical device that can help patients adhere to prescribed dosages by controlling access to their medication and dispensing it properly.
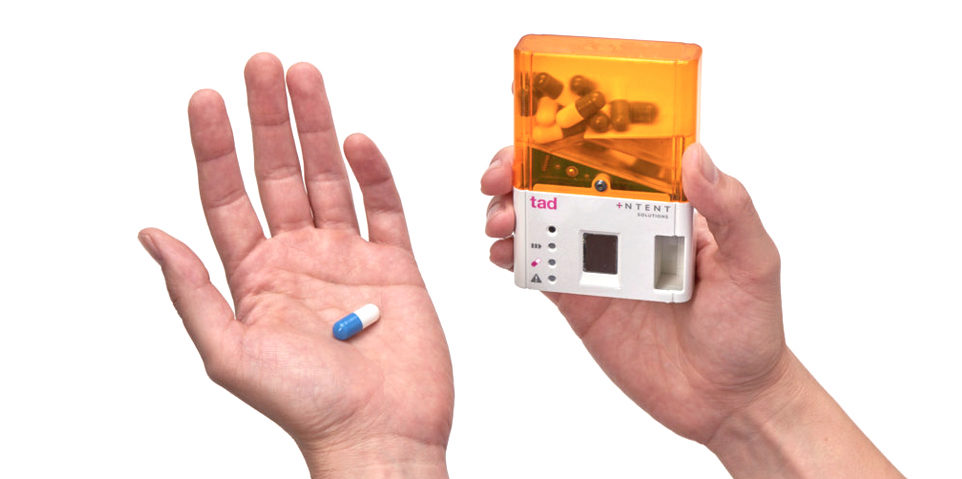
In 2013, the team founded the Atlanta-based company Intent Solutions, whose iPhone-sized smart-dispenser alerts users when it’s time to take their painkillers and only dispenses the amount prescribed via thumbprint sensor, never giving anyone access to the entire contents of the medication. The device collects and transmits adherence data via the cloud to monitoring organizations and caregivers. It can detect too frequent attempts to access pills or if anyone other than the patient is trying to reach the medication stored in the tamper-resistant device.
“Even though the opioid crisis may not have been as broadly known then as it is today, once we dug in and did the research, the problem was evident, with people dying every day and tremendous associated costs,” says Hancock, a strategic marketing professional who earned his BS from the School of Chemical & Biomolecular Engineering in 1990.
According to the National Institute on Drug Abuse, more than 130 deaths a day in the United States are due to opioid overdoses from prescription pain relievers, heroin, and synthetic opioids such as fentanyl. The crisis of opioid misuse costs the United States $78.5 billion a year in health care expenses, lost productivity, and criminal justice. Of patients prescribed opioids for chronic pain, 21 to 29 percent misuse them, and between 8 and 12 percent develop an opioid use disorder.
Market Makers
While a device to control dispensing might sound like a “no brainer,” Hancock says, there was nothing like it on the market at the time. “We’re market makers,” he says.
Currently, Intent Solutions’ device is in pilot studies with patients in Hospice, in clinical trials, and on workers' compensation. Ultimately, Hancock believes that this device could be applicable to almost any medication, especially those where adherence is crucial, such as blood pressure, post-transplant, and cancer drugs.
“Once we collect enough data to prove our value proposition, it will make sense to health care providers and insurers and really take off,” Hancock believes. “Adoption will increase and accelerate.”
After co-founding Intention Solutions, Hancock held roles including chief executive officer, chief operating officer and chief financial officer, but last year the company completed its executive team, and he stepped away from day-to-day operations to focus on other promising medical device technologies. He runs Innovetica, which he founded in 2009 as a technology incubation firm, and from which Intent Solutions evolved.
At Innovetica, he works closely with physician and research inventors to develop products, managing the whole process from idea generation and market research to engineering and product development to intellectual property and regulatory issues.
Moving from Petrochemicals to Medical Devices
After graduating from Tech, Hancock went to work for Amoco in a petrochemical plant in Decatur, Alabama, and later transferred to Chicago, Illinois, after the BP Amoco merger. During his 16 years with the company, he held various leadership positions with oversight for engineering, manufacturing, operations planning, and supply chain optimization.
While in Chicago, he earned his MBA at the Kellogg School of Management at Northwestern University, an education that helped him develop the expertise to transition into medical devices. “That space is all about new products and improving health. It’s fast-paced and creative,” he says.
After finishing his MBA in 2006, he returned to Atlanta to join C.R. Bard, where he held marketing leadership positions of increasing responsibility for an extensive surgical implant portfolio. From 2009 to 2010, he managed the medical device portfolio for EndoChoice, an Atlanta-based startup that since had gone public, then was acquired by Boston Scientific. In addition, he served as executive director of the Southeastern Medical Device Association (SEMDA) from 2011 to 2013.
Through his incubation firm, he’s co-founded another company, Park Surgical Innovations, which has developed a surgical assistive device for laparoscopic hernia repair that will soon be ready to submit to the Food and Drug Administration for approval. Other projects are in earlier developmental stages in interventional cardiology and the repurposing of existing therapeutic entities.
Hancock, who was inducted into Georgia Tech College of Engineering’s Academy of Distinguished Alumni in 2018, credits his chemical engineering education for developing the critical thinking skills he uses every day in his current field.
He believes that for each project you should build a company with the intent of commercializing, instead of simply licensing the technology. A license agreement may ultimately be the outcome, but an appropriate infrastructure would have to be established, creating more value for potential acquirers, he says.
Outside of work, Hancock keeps busy as the father of a six-year-old daughter and enjoys Georgia Tech athletics, backpacking, biking, skiing, and live music. He met his wife, Elizabeth, in Dublin, Ireland, in 2016, when Georgia Tech opened the football season against Boston College there. She is also a Tech grad who earned her BS in computer science in 1996. “We lived just a couple miles apart in Atlanta, but didn’t know each other. We had each traveled to Dublin separately to meet friends. We married a year later to the day.”